Documents
Photoinduced reactions for a green synthesis of bioactive molecules
Faïza Diaba studied Chemistry at the University of Constantine, Algeria. She then obtained her PhD in Organic Chemistry with Dr. Micheline Grignon Dubois at Bordeaux I University, France. After a two years Postdoctoral position with Prof. Josep Bonjoch at Barcelona University, she pursued her scientific career as a Lecturer and then as an Associate Professor at the same university. Her research interests are focused on developing new synthetic methodologies for the synthesis of bioactive molecules using radical, photoredox and green chemistry.
For more information visit:
https://orcid.org/0000-0001-9950-2470
https://www.scopus.com/authid/detail.uri?authorId=6602286833
Synthesis of bioactive molecules using green chemistry where we aim to use accessible natural resources like light, recycled materials, non-contaminating reagents and solvents with almost zero waste.
Research project history
The COVID-19 pandemic has had a significant impact on our lives. The virus, which first emerged in Wuhan, China in 2019, quickly spread around the world, leading to widespread lockdowns and other measures to try to slow the spread of the virus. These measures have had a profound effect on our daily lives, with many people losing their jobs, businesses shutting down, and schools and universities moving to online learning. Nevertheless, the COVID-19 pandemic has had a significant impact on the environment and people's attitudes towards ecology. With lockdowns and travel restrictions in place, there has been a decrease in air and noise pollution, leading to improvements in air and water quality. Additionally, the pandemic has brought attention to the importance of environmental issues and the need for sustainable solutions.
As synthetic chemists, our daily work involves dealing with potential hazards and risks, particularly when it comes to manipulation of toxic or/and flammable chemicals. Moreover, synthesis of organic compounds generates several and high amount of contaminant residues and waste, in figure 1 are presented some of the typical residues and waste generated in a laboratory of organic synthesis.
Figure 1. Sample of residues and waste generated in an organic synthesis laboratory (plastic syringes, nitrile butadiene rubber gloves, halogenates and non-halogenated residues).
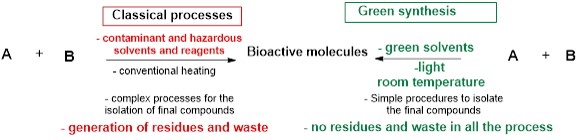
The covid period allowed me to think about using green chemistry to achieve reactions in a more efficient and ecological way. Green (Sustainable) chemistry is a branch of chemistry that focuses on the development of processes and products that are environmentally friendly and socially responsible. This includes the use of renewable resources, the minimization of waste and pollution, and the design of products with a reduced environmental impact. Examples of sustainable chemistry practices include the development of new catalysts for more efficient chemical reactions, take advantage on available natural sources namely light to activate reactions and the design of safer and more biodegradable products and solvents. The goal of green chemistry is to create a more sustainable future by reducing the negative impact of chemical production and use on the environment and human health.
Figure 2. Main differences between green and classical chemistry.
Cancer is a leading cause of death worldwide, nearly 10 million deaths in 2020 (nearly one in six deaths) with an estimated 18.1 million new cases according to world health organization. The cancer rates in Spain are appalling. In 2020 alone, there were more than 282,000 new cancer cases and over 113,000 cancer deaths. Currently, the five most common types of cancer that have devastated the Spanish population are: colorectal, prostate, breast, Lung and Bladder cancers.
There are over 100 different types of cancer, and each one requires different treatments. Moreover, Cancer cells can develop resistance to existing treatments over time, making it difficult to effectively treat the disease. Hence, research and development of new targets for cancer treatment is an active field, and scientists are constantly working to prepare new molecules for treatment and also on developing new therapies.
Current investigation
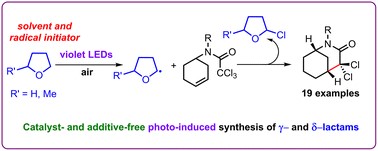
Based on the background reported previously, about 5 years ago we started a new investigation project aiming the synthesis of bioactive molecules using sustainable green synthetic procedures. Hence the first photoinduced reaction was successfully carried out, in the presence as well as in the absence of a photocatalyst, leading to the first preparations of lactams from N-alkenyl-tethered trichloroacetamides. Additionally, the prepared lactams as well as other prepared in another context, were tested for their cytotoxic activity showing an interesting selectivity against squamous carcinoma cells (A431).
- Photoredox catalysis in the synthesis of γ- and δ-lactams from N-alkenyl trichloro- and dichloroacetamides, G. Trenchs, F. Diaba, Org. Biomol. Chem., 2022,20, 3118-3123. DOI: https://doi.org/10.1039/D2OB00276K

- Catalyst-free photo-induced aerobic radical synthesis of lactams from N-alkenyl trichloroacetamides in 2-methyltetrahydrofuran as the radical initiator under violet light, F. Diaba, G. Trenchs, Org. Biomol. Chem., 2024, 22, 388-394. DOI: https://doi.org/10.1039/D3OB01804K
- Microwave-Assisted Atom Transfer Radical Cyclization in the Synthesis of 3,3-Dichloro-γ- and δ-Lactams from N-Alkenyl-Tethered Trichloroacetamides Catalyzed by RuCl2(PPh3)3 and Their Cytotoxic Evaluation, F. Diaba; A. G. Sandor; M. d. C. Morán, Molecules 2024, 29(9), 2035. https://doi.org/10.3390/molecules29092035
- Cytotoxic Assessment of 3,3-Dichloro-β-Lactams Prepared through Microwave-Assisted Benzylic C-H Activation from Benzyl-Tethered Trichloroacetamides Catalyzed by RuCl2(PPh3)3. F. Diaba; A. G. Sandor; M. d. C. Morán, Molecules, 2022, 27, 5975. https://doi.org/10.3390/molecules27185975olecules 2022, 27, 5975. https://doi.org/10.3390/molecules27185975
Projects:
Photocatalysis in C-C and C-heteroatom bond formation towards the synthesis of natural compounds (IP: Faïza Diaba), Fundació Bosch i Gimpera (Barcelona, ES), 2020-2026.
Faïza DIABA
Laboratori de Química Orgànica
Departament de Farmacologia, Toxicologia i Química Terapèutica
Facultat de Farmàcia
Av. Joan XXIII, 27-31
08028 Barcelona
Tel. 934035849 ex 118
Email: faiza.diaba@ub.edu