The usual formulation of thermodynamics rests on the assumption of additivity, in the sense that thermodynamic potentials are linear homogeneous functions of extensive variables. The corresponding description on the grounds of statistical mechanics reflects this assumption as well. Due to additivity, extensive quantities are linear functions of the system size and the thermodynamic potentials present always the same concavity. Macroscopic systems with short-range interactions are additive. In contrast, systems with a small number of particles (small systems), systems with confinement imposed by external conditions and systems with long-range interactions are, in general, not additive. In the case of small systems, additivity and the usual thermodynamics of macroscopic systems are recovered when the number of particles in the system goes to infinity, provided the range of the (short-range) interactions becomes negligible with respect to the system size. The situation changes, however, for confined systems and for macroscopic systems with long-range interactions in which the number of particles can be large in a proper thermodynamic limit. Roughly speaking, a system is nonadditive when the interactions are comparable with the system size, and the effects of nonadditivity can be expected to play a significant role in the statistical mechanics and thermodynamics frameworks. Remarkably, typical features of nonadditive systems are the occurrence of negative resposte functions, ensemble inequivalence, and the possibility of observing equilibrium states under completely open conditions. Below we provide more details by discussing some selected results of our research in this field.
Generalized Gibbs-Duhem equation for long-range systems
The Gibbs-Duhem equation describes the relation between variations of chemical potential, pressure and temperature of the system in equilibrium conditions. One important consideration that must be taken into account when dealing with nonadditive systems is that the usual version of this equation does not hold in general. In particular, a generalization was derived in [1] for systems with strong long-range interactions in which the interaction potential decays slower than the dimension of the embedding space. Due to interactions, the distribution of particles in these systems is usually not uniform and the potential energy scales with the square of the number of particles, unlike macroscopic systems with short-range interactions. As shown in [1], it is precisely an additional contribution depending on the long-range potential energy which modifies the Gibbs-Duhem equation for these systems.
Thermodynamics of nonadditive systems
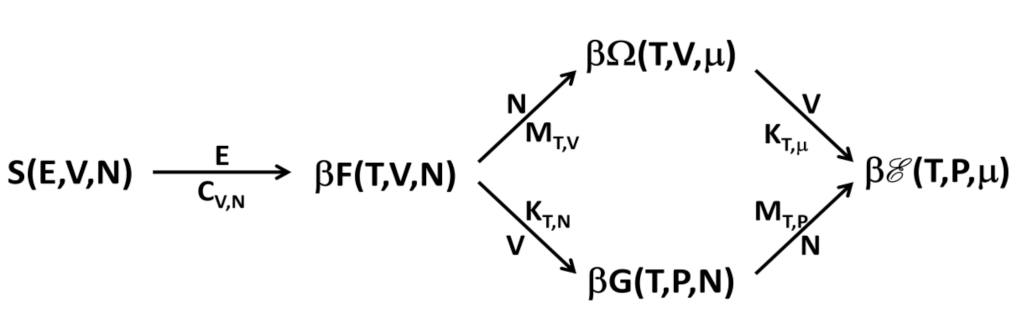
An adequate framework for the thermodynamics of a nonadditive system can be justified by formulating the problem at the level of an ensemble of virtual replicas of the system. Since the replicas do not interact with each other and their number can be arbitrarily large, the ensemble itself is an additive system. Thus, the usual thermodynamic treatment can be used to describe the ensemble if the number of replicas is incorporated as an additional variable together with its conjugate variable, the replica energy. Once the problem is formally solved for the ensemble, properties of the single system are obtained as ensemble properties per replica, so actually the number of replicas plays no role. The replica energy, however, turns out to be a property of the system and it does play an important role. Being a system property, the replica energy can be computed for the case under consideration, typically by resorting to the methods of statistical mechanics. The system is then nonadditive when the replica energy is different from zero, since in this case the thermodynamic potentials are not linear homogeneous functions of extensive variables. In his Thermodynamics of small systems, Hill showed that the replica energy (or subdivision potential) does not vanish when the system has a small number of particles. In [2], we showed that the replica energy is, in general, different from zero for long-range interacting systems with a large number of particles, in a proper thermodynamic limit. A confined system was considered in [6] showing the same feature, highlighting the generality of this approach. Notably, the scaling properties of the entropy in nonadditive systems are quite different from those of usual macroscopic systems with short-range interactions.
Statistical mechanics and thermodynamics of nonadditive systems
The near-field radiative heat transfer between objects can also be controlled by the action of external fields. An interesting example is what happens with magneto-optical materials like InSb, where dipolar resonances can show a significant dependence on an applied magnetic field. Hence, the heat flux emitted by a magneto-optical particle can be dramatically altered by tuning this applied field. On account of this fact, a new thermomagnetic effect was predicted in [4]: a magnetoresistance for the heat flux carried by thermal photons. As shown in the accompanying figure, thermal resistance variations of about 50% along chains of InSb-Ag nanoparticles were anticipated for fields of a magnitude of about 500 mT. Moreover, for these chains at room temperature, the resistance can be increased by almost a factor of 2 with magnetic fields of 2 teslas. As discussed in [4], this thermal magnetoresistance results from a strong spectral shift of localized surface waves supported by the particles under the action of the magnetic field. This effect is promising for practical applications, especially in the field of thermal management at the nanoscale as well as for magnetic sensing with temperature or heat flux measurements.
Concavity properties of the replica energy
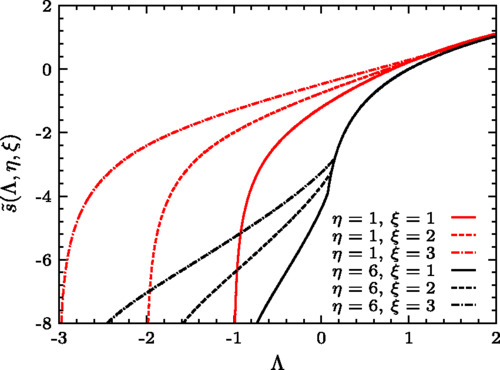
In general, it can be shown that the replica energy explicitly enters in the thermodynamic formalism by modifying the Gibbs-Duhem equation, and the usual version of this equation is obtained for cases where the replica energy vanishes. It follows that the natural variables for the replica energy are the chemical potential, pressure and temperature, which can be independent if the system is nonadditive. As pointed out by Hill for small systems, the replica energy is the relevant free energy from which the thermodynamics must be derived when chemical potential, pressure and temperature are the relevant control parameters. It was shown in [3] for long-range interacting systems and generally in [4] without model assumptions that this is also the case in the limit of large number of particles. As for any other free energy, the replica energy can be related to the entropy by Legendre-Fenchel transformations [4], a generalization of the usual Legendre transformation that applies even in the case of nonconvex entropies. The occurrence of curvature anomalies in the entropy or in the usual thermodynamic potentials is a salient property of nonadditive systems and is related to ensemble inequivalence. The replica energy, in contrast, is always a completely concave function in terms of its natural variables [4].
Phase transitions in the unconstrained ensemble
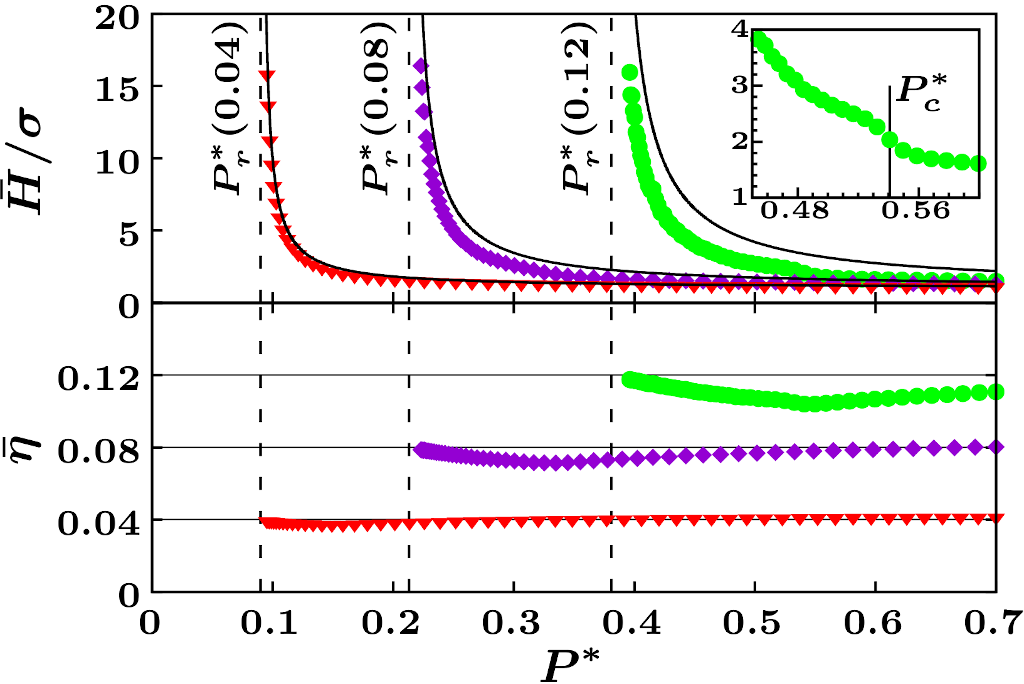
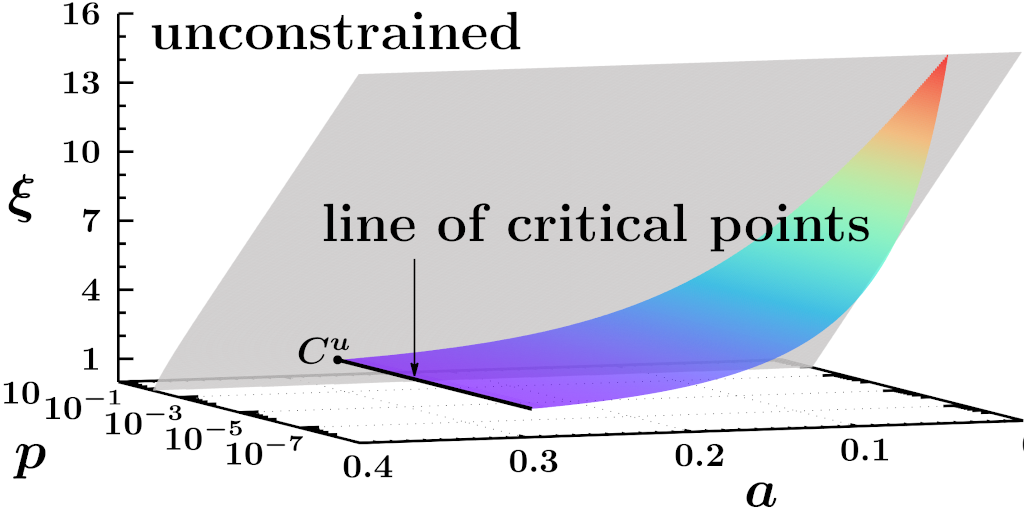
Completely open systems can exchange heat, work, and matter with the environment. While energy, volume, and number of particles fluctuate under completely open conditions, the equilibrium states of the system, if they exist, can be specified using the temperature, pressure, and chemical potential as control parameters. The unconstrained ensemble [3] is the statistical ensemble describing completely open systems and the replica energy, as mentioned above, is the appropriate free energy for these control parameters from which the thermodynamics must be derived. It turns out that macroscopic systems with short-range interactions cannot attain equilibrium configurations in the unconstrained ensemble, since temperature, pressure, and chemical potential cannot be taken as a set of independent variables in this case. In contrast, we shown that systems with long-range interactions [3] and confined systems [6] can reach states of thermodynamic equilibrium in the unconstrained ensemble. Furthermore, in [5] we demonstrated that not only equilibrium states can exist in this ensemble, but also that completely open systems can undergo first-order phase transitions. This was shown by studying a modified version of the Thirring model with attractive and repulsive interactions and with particles of finite size. The model exhibits first-order phase transitions in the unconstrained ensemble, at variance with the analogous model with point-like particles. A phase diagram in the unconstrained ensemble is represented in the accompanying figure.
Monte Carlo simulations of completely open systems
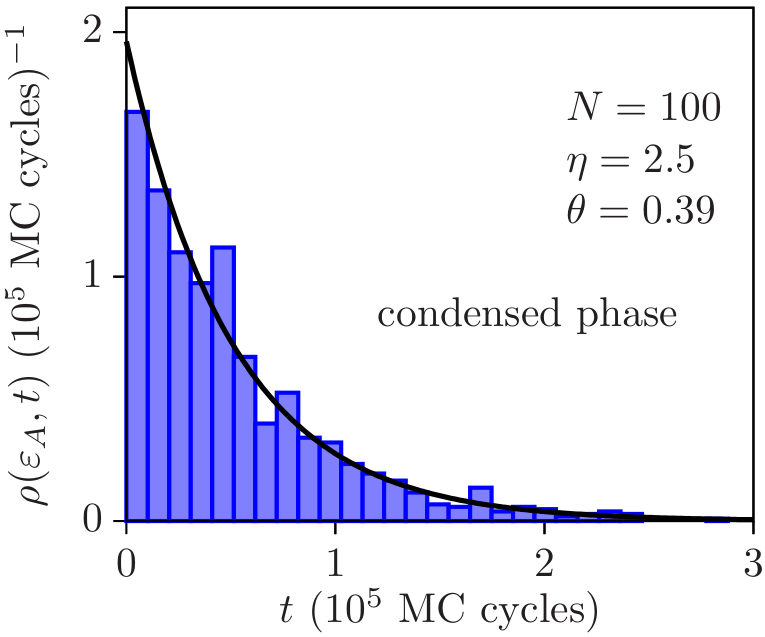
In [6], we proposed a method to perform Monte Carlo simulations in the unconstrained ensemble and discussed when equilibrium states can be expected under completely open conditions. In a thermodynamic description, the surroundings of a completely open system remove the constraints on energy, volume, and matter content by imposing fixed temperature, pressure, and chemical potential. The latter cannot be taken together as independent control parameters when the system is macroscopic and when interactions are short-ranged. However, if the system is either small due to a reduced number of particles or to confinement, or is macroscopic but has long-range interactions, this premise changes because the system becomes nonadditive, which leads to the appearance of an additional degree of freedom that may render temperature, pressure, and chemical potential an independent set of control parameters. In [6] we examined two simple physical situations, a long-range interacting system and a confined hard-sphere fluid, and analyze how nonadditivity gives rise to equilibrium states under completely open conditions. The proposed method could open new avenues for simulations of systems that exchange heat, work and matter with their environment.
References
[1] I. Latella and A. Pérez-Madrid. Local thermodynamics and the generalized Gibbs-Duhem equation in systems with long-range interactions. Phys. Rev. E 88, 042135 (2013); https://doi.org/10.1103/PhysRevE.88.042135; https://arxiv.org/abs/1302.4903
[2] I. Latella, A. Pérez-Madrid, A. Campa, L. Casetti, and S. Ruffo. Thermodynamics of nonadditive systems, Phys. Rev. Lett. 114, 230601 (2015); https://doi.org/10.1103/PhysRevLett.114.230601; https://arxiv.org/abs/1505.03767
[3] I. Latella, A. Pérez-Madrid, A. Campa, L. Casetti, and S. Ruffo. Long-range interacting systems in the unconstrained ensemble, Phys. Rev. E 95, 012140 (2017); https://doi.org/10.1103/PhysRevE.95.012140; https://arxiv.org/abs/1611.05694
[4] A. Campa, L. Casetti, I. Latella, A. Pérez-Madrid and S. Ruffo. Concavity, Response Functions and Replica Energy, Entropy 20(12), 907 (2018); https://doi.org/10.3390/e20120907; https://arxiv.org/abs/1810.11309
[5] A. Campa, L. Casetti, I. Latella and S. Ruffo. Phase transitions in the unconstrained ensemble, Journal of Statistical Mechanics: Theory and Experiment 014004 (2020); https://doi.org/10.1088/1742-5468/ab6098; https://arxiv.org/abs/1910.13997
[6] I. Latella, A. Campa, L. Casetti, P. Di Cintio, J. M. Rubi and S. Ruffo. Monte Carlo simulations in the unconstrained ensemble, Phys. Rev. E 103, L061303 (2021); https://doi.org/10.1103/PhysRevE.103.L061303; https://arxiv.org/abs/2104.06103
