
First-order phase transitions play an important role in science, nature and many technical applications. Simple, everyday examples are condensation, evaporation, crystallization, and melting. As the first step, all these first-order phase transitions need to overcome a free-energy barrier, which is the work of formation of a small embryo or nucleus of the new phase. This nucleus can only emerge from random thermal fluctuations within the old metastable phase. This initiating process of a first-order phase transition is called nucleation.
Apart from these everyday examples, nucleation is vital in many other fields: In biophysics, the nucleation of bubbles in the DNA is essential for its replication and transcription. The crystallization of proteins is instrumental in the building of their structure and in the development of new drugs. It is behind many diseases such as sickle-cell anemia or Alzheimer. A fundamental step in the replication of a virus is the self-assembly of its rigid shell (capsid) from its proteins, which may also be described as a nucleation mechanism. Bubble nucleation and crystallization are important processes in polymers. In microemulsions, we encounter micellar formation and liquid-liquid phase transitions, e.g. from a bicontinuous sponge to a micellar oil-in-water phase. The formations of black holes, volcano eruptions, and the popular Diet-Coke-and-Mentos experiment are further examples involving a nucleation step.

Homogeneous and heterogeneous nucleation
Heterogeneous nucleation begins on alien surfaces or particles, or pre-existing nuclei in the old phase. Dust or atmospheric aerosols can serve as heterogeneous nucleation centers for water condensation in the atmosphere. Likewise, the microscopically rough surface of a Champagne glass offers nucleation sites for CO2 bubbles (see picture above). We can do a simple “kitchen-sink” experiment to demonstrate this by clensing the Champange glass with dish-washing detergent before pouring the Champagne into the empty but still wet glass. Noticeably less bubbles appear in the glass because the water-detergent coating on the glass effectively smoothes out the roughness of its surface.
We can also induce heterogeneous nucleation in a one-component system by seeding nuclei of the new phase into the metastable old phase. For instance, we can easily trigger the crystallization of a saturated saline solution or a supercooled liquid by adding just a few grains of the salt to the solution. In the following video, this is demontrated by dropping a few ice-crystals into a bottle of supercooled water, which then completely freezes within seconds:
Heterogeneous nucleation typically has a much smaller barrier towards the phase transition and most phase transitions occurring in experiments or nature are started by heterogeneous nucleation. Still, the focus in nucleation research often lies on the underlying homogeneous nucleation: the formation of the new phase solely from fluctuations within the old phase.
Simplest example: condensation
The formation of a small liquid droplet in a supersaturated vapor (see Figure below) is well accessible by experiments and theoretical approaches, and of fundamental interest in atmospheric sciences
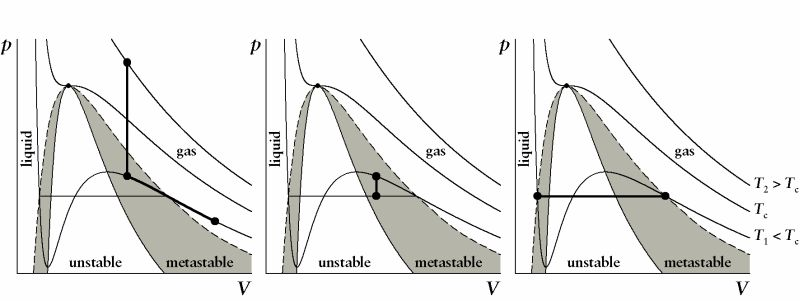
We can compress the vapor at constant temperature and the condensation will not commence at the saturation pressure. The vapor remains in a metastable state for some time until thermal fluctuations form a sufficiently large cluster (a nucleus), which then can grow on spontaneously. In this case, the barrier towards the condensation is the work of formation for building the curved surface of a small liquid droplet inside the vapor.
The formation of clouds in the atmosphere, essentially the condensation of water vapor, is such an example.
