8 August, 2023
The role of huntingtin (Basic Research)

THE SOLUBLE MUTANT HUNTINGTIN PROTEIN IS ONE OF THE RESPONSIBLE FOR THE SPREADING AND DEVELOPMENT OF HUNTINGTON'S DISEASE IN THE EARLY STAGES
The pioneering research carried out by the Stem Cells and Regenerative Medicine research group of the Basic and Translational Research Area of Creatio – University of Barcelona, demonstrates the crucial role played by the soluble form of the mutated huntingtin protein in triggering Huntington’s disease in the initial stages. The mutated huntingtin protein interacts with cellular components and promotes the development of Huntington’s disease. When affected human neurons are transplanted into mouse brains, the cells spread the disease to healthy neurons due to the transfer of the mutated human protein. These findings show a hopeful future in the development of new therapeutic strategies to treat Huntington’s disease and offer a better future for new generations that suffer from the disease.
Huntington’s disease (HD) is a neurodegenerative disease that results from a mutation in the DNA sequence that encodes the protein huntingtin (HTT). This mutation in the DNA consists of an expansion of the CAG sequence, more than 40-fold, resulting in a mutated protein (mHTT) with incorrect folding. mHTT loses its function and stability and induces progressive neuronal death. This progressive death causes patients to have involuntary movements, a lack of coordination, and loss of mobility, among other symptoms. As the disease progresses, symptoms become more severe, leading to complete loss of walking, speech, and mood swings, among others.
Currently, HD presents an unmet medical need there is no cure or effective treatment to address the disease. Patients only have palliative treatments to relieve symptoms or slow progression. That is why it is important and essential to study the underlying mechanisms of the disease and identify effective therapeutic targets to address it.
The study carried out by the Stem Cells and Regenerative Medicine research group of the Basic and Translational Research Area of Creatio – University of Barcelona, in collaboration with the Germans Trias i Pujol Research Institute (IGTP), the Environmental and Technological Energy Research Center (CIEMAT) and Cardiff University (UK) have studied the contribution of mHTT in disease’s propagation and its interaction with key cellular organelles (mitochondria, endoplasmic reticulum, nuclear membrane). The results were recently published in the scientific journal Cellular and Molecular Life Science.
Currently, animal models that are available to study HD do not correctly reproduce the disease; the number of CAG repeats, and the development and progression of the disease are different between both species. An alternative to study HD would be to analyse it directly in the affected brain tissue of patients. However, in those cases, the disease has already started and is in initial and/or advanced stages. In addition, there are ethical and access limitations. These factors make the research difficult. To better reproduce the human conditions of the disease and obtain representative results with a high success rate for future clinical studies, the scientific community is committed to using human stem cells derived from HD patients and transplanting them into the brains of animal models. This allows us to study more precisely the effect that the mHTT protein triggers when it interacts with healthy cells.
The results of our study show that the human mHTT protein interacts with cellular components, endoplasmic reticulum, mitochondria, and/or nuclear membrane, and induces structural and functional alterations in them. To mitigate the damage, human cells secrete the mHTT protein, and transfer it to healthy mouse cells, thereby inducing the development of the disease.
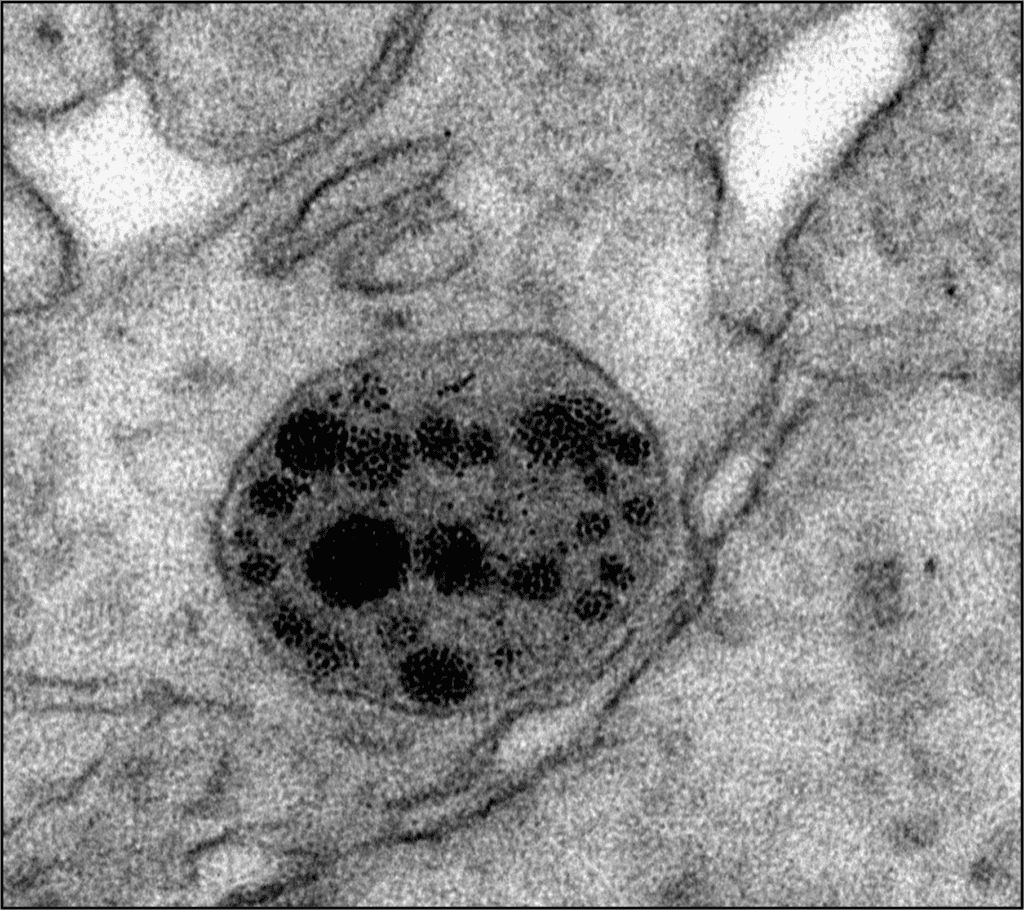
This research helps clarify and resolve questions about the role of the mHTT protein in the progression of HD and the mechanism of propagation between cells. The researchers mention that these findings will provide new therapeutic targets for developing effective strategies to treat Huntington’s disease.
This study was financed by public grants from the Ministry of Science, Innovation and Universities and the European Regional Development Fund (ERDEF); the Carlos III Health Institute, Ministry of Science, Innovation and Universities and ERDF [CIBERNED and RETICS (Cell Therapy Network)]; the Government of Catalonia; “la Caixa” Foundation; and CHDI Foundation Inc. (USA).
Article: Miguez, A., Gomis, C., Vila, C. et al. Soluble mutant huntingtin drives early human pathogenesis in Huntington’s disease. Cell. Mol. Life Sci. 80, 238 (2023). https://doi.org/10.1007/s00018-023-04882-w