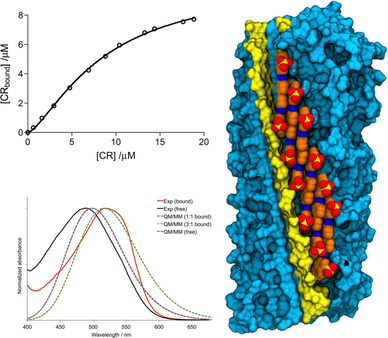
In humans, amyloid aggregation is related to up to thirty-six degenerative protein misfolding disorders, including non-neurologic and neurologic illnesses, such as type 2 diabetes or Alzheimer’s and Parkinson’s diseases. Amyloids are characterized by their capacity to bind Congo red (CR), one of the most used amyloid-specific dyes. The structural features of CR binding were unknown for years. In this study, we combined spectroscopic data with molecular docking, molecular dynamics, and excitonic quantum/molecular mechanics calculations to examine and rationalize CR binding to amyloids concluding that CR binds to amyloid fibrils with the long axis oblique to the fibril axis and the plane radial to the fibril core, following a cooperative mechanism characterized by the formation of a 1:1 stoichiometric complex. For more information check this link.