Energy transfer in photosynthesis and other biosystems

Electronic energy transfer is often modeled using Förster theory, developed more than 60 years ago, which describes an energy hopping mechanism mediated by dipole-dipole interactions between the interacting chromophores. Whereas this description is well-suited for a variety of systems, recent evidence have unveiled the many interesting subtleties of closed packed multichromophoric aggregates that go beyond Förster theory, including coherence effects, deviations from the dipole approximation or environment screening effects. In this context, we work in the development of accurate multiscale methods aimed at describing such processes in molecular detail.
Selected references:
1) L. Cupellini, M. Corbella, B. Mennucci and C. Curutchet, Electronic energy transfer in biomacromolecules, WIREs Comput Mol Sci. 2019, 9(2), e1392.
2) C. Curutchet and B. Mennucci, Quantum Chemical Studies of Light Harvesting, Chem. Rev. 2017, 117 (2), 294–343.
3) C. Curutchet, J. Kongsted, A. Muñoz-Losa, H. Hossein-Nejad, G. D. Scholes and B. Mennucci, Photosynthetic light-harvesting is tuned by the heterogeneous polarizable environment of the protein, J. Am. Chem. Soc. 2011, 133(9), 3078-3084.
4) C. Curutchet and A. A. Voityuk, Triplet–Triplet Energy Transfer in DNA: A Process That Occurs on the Nanosecond Timescale, Angew. Chem. Int. Ed. 2011, 50(8), 1820-1822.
5) D. L. Andrews, C. Curutchet and G. D. Scholes, Resonance Energy Transfer: Beyond the Limits, Laser & Photonics Rev. 2011, 5, 114-123.
6) D. Beljonne, C. Curutchet, G. D. Scholes and R. J. Silbey, Beyond Förster resonance energy transfer in biological and nanoscale systems, J. Phys. Chem. B Feature Article, 2009, 113(19), 6583–6599.
Hole transfer in DNA
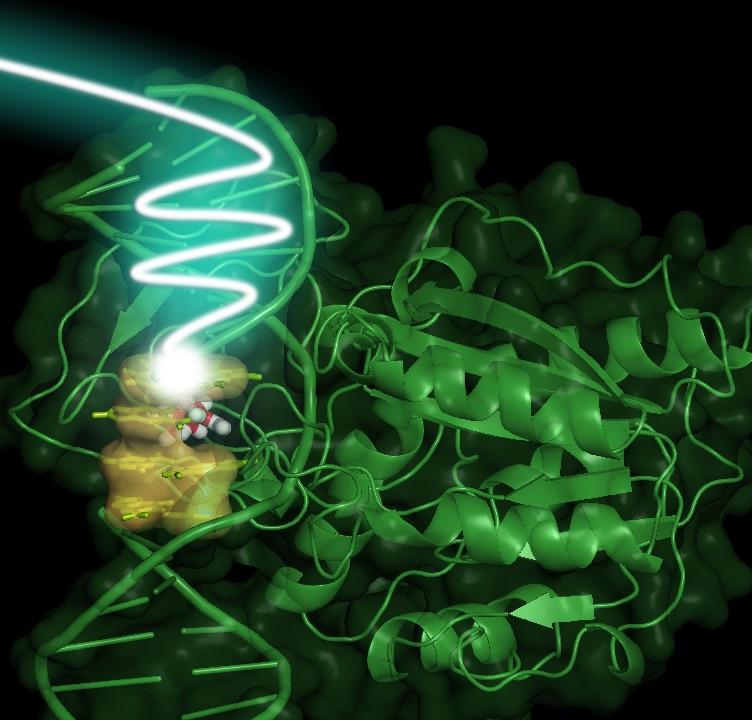
DNA lesions or the interaction with proteins can drastically modulate HT dynamics in DNA
There are still many open questions related to the behaviour of hole transfer (HT) reactions in DNA, with implications in DNA oxidative damage and the use of HT to sense DNA repair enzymes. In this research line, we aim at understanding how such processes are modulated by the environment, for example when the double helix interacts with proteins or in the presence of DNA lesions.
Selected references:
1) M. Corbella, A. A. Voityuk and C. Curutchet, How abasic sites impact hole transfer dynamics in GC-rich DNA sequences, Phys. Chem. Chem. Phys. 2018, 20(35), 23123–23131.
2) M. Corbella, A.A. Voityuk and C. Curutchet, Single Amino Acid Mutation Controls Hole Transfer Dynamics in DNA-Methyltransferase HhaI Complexes, J. Phys. Chem. Lett. 2015, 6(18), 3749–3753.
Continuum solvation models
The description of solvent (or more generally enviromnent) effects is key in condensed phase chemistry. We develop quantum mechanical continuum solvation models for the description of solvent effects in a variety of applications, from the prediction of solvation free energies to the study of solvatochromic effects in excited states, or the investigation of screening effects in electronic energy transfer.
Selected references:
1) W. J. Zamora, C. Curutchet, J. M. Campanera and F. J. Luque, Prediction of pH-Dependent Hydrophobic Profiles of Small Molecules from Miertus−Scrocco−Tomasi Continuum Solvation Calculations, J. Phys. Chem. B 2017, 121(42), 9868–9880.
2) A. Klamt, B. Mennucci, J. Tomasi, V. Barone, C. Curutchet, M. Orozco and F. J. Luque, On the Performance of Continuum Solvation Methods. A Comment on Universal Approaches to Solvation Modeling, Acc. Chem. Res., 2009, 42(4), 489–492.
3) G.D. Scholes, C. Curutchet, B. Mennucci, R. Cammi and J. Tomasi, How solvent controls electronic energy transfer and light harvesting, J. Phys. Chem. B 2007, 111(25), 6978-6982.
4) C. Curutchet, M. Orozco, F. J. Luque, B. Mennucci and J. Tomasi, Dispersion and repulsion contributions to the solvation free energy: Comparison of quantum mechanical and classical approaches in the polarizable continuum model, J. Comput. Chem. 2006, 27(15), 1769-1780.
5) C. Curutchet, C. J. Cramer, D. G. Truhlar, M. Ruiz Lopez, D. Rinaldi, M. Orozco and F.J. Luque, Electrostatic Component of Solvation: Comparison of SCRF Continuum Models, J. Comput. Chem. 2003, 24(3), 284-297.
6) C. Curutchet, M. Orozco and F.J. Luque, Solvation in Octanol: Parametrization of the Continuum MST Model, J. Comput. Chem. 2001, 22(11) 1180-1193.
Polarizable QM/MM models
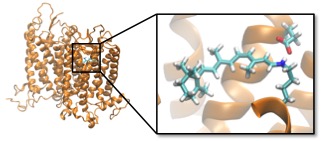
We develop polarizable quantum/molecular mechanics (QM/MMpol) approaches to describe environment effects with explicit account for environment MM polarization. QM/MM methods allow an efficient description of the enviroment through classical force fields, while still describing quantum-mechanically the molecules or parts of the system where we want to focus our attention. Such strategy is specially convinient in highly heterogeneous enviromnents like proteins, where a continuum dielectric description obscures the often critical role of local molecular structure.
Selected references:
1) C. Curutchet, L. Cupellini, J. Kongsted, S. Corni, L. Frediani, A. H. Steindal, C. A. Guido, G. Scalmani and B. Mennucci, Density-Dependent Formulation of Dispersion−Repulsion Interactions in Hybrid Multiscale Quantum/Molecular Mechanics (QM/MM) Models, J. Chem. Theory Comput. 2018, 14(3), 1671–1681.
2) Q. Li, B. Mennucci, M. A. Robb, L. Blancafort and C. Curutchet, Polarizable QM/MM Multiconfiguration Self-Consistent Field Approach with State-Specific Corrections: Environment Effects on Cytosine Absorption Spectrum, J. Chem. Theory Comput. 2015, 11(4), 1674-1682.
3) S. Caprasecca, C. Curutchet and B. Mennucci, Toward a Unified Modeling of Environment and Bridge-mediated Contributions to Electronic Energy Transfer: a Fully Polarizable QM/MM/PCM Approach, J. Chem. Theory and Comput. 2012, 8(11) 4462-4473.
4) C. Curutchet, A. Muñoz-Losa, S. Monti, J. Kongsted, G. D. Scholes and B. Mennucci, Electronic energy transfer in condensed phase studied by a polarizable QM/MM model, J. Chem. Theory Comput. 2009, 5(7), 1838-1848.