|
StroomaLab: Translational Microenvironment Research in Lung
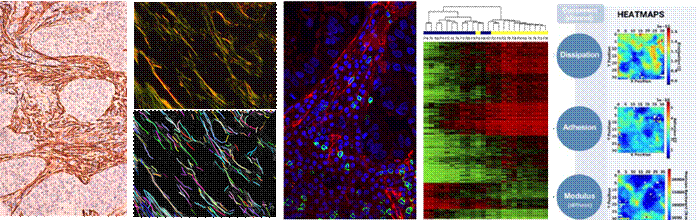
Cancer and Lung Fibrosis Research interests overview
The stroma is the connective tissue
rich in fibroblasts and a collagenous extracellular matrix (ECM) that
surrounds epithelial cells and provides key biomechanical and biochemical cues
to guide normal development, repair and tissue-specific functions.
Conversely, stromal composition and associated signaling becomes chronically
awry in prevalent and fatal diseases like cancer and organ fibrosis. In the
context of the lung, a hallmark of both lung cancer and pulmonary fibrosis is
a persistent desmoplastic stroma rich in activated fibroblasts/myofibroblasts
in the background of an excessive deposition of fibrillar collagens, which has
been implicated in virtually all steps of disease progression and resistance
to therapies. Our lab aims to understand the origins of the pathologic
fibrotic stroma both in the context of the primary tumor and brain metastasis,
how it contributes to disease progression, how can it be targeted
therapeutically, and how can it be used to identify clinically-relevant
biomarkers. For this purpose, we combine cutting-edge preclinical models
(in culture, in vivo and ex-vivo) based on patient-derived samples (including
primary fibroblasts and tissue samples from patients with lung cancer or
idiopathic pulmonary fibrosis), state-of-the-art molecular and cell
biology techniques (including “omics”), and bioengineering approaches
(mainly atomic force microscopy, microfluidics and digital pathology). We
then apply these tools to study quantitatively the aberrant interactions
between fibroblasts and other stromal cells (immune cells, endothelial cells)
or epithelial cells as well as the aberrant collagenous ECM and associated
mechanobiology. Our ultimate goal is to be translational and bring our knowledge to clinical settings, and
to this aim we have ongoing collaborations with clinical groups and
collaborate with companies to check the suitability of novel drugs against
the aberrant stroma as well as novel biomarker for improve diagnosis,
prognosis and therapeutic guidance.
For more specifics about our past and
current research, please browse the different
sections below: [LAB MEMBERS] [RESEARCH] [PUBLICATIONS] [COLLABORATION WITH INDUSTRY]
[NEWS&VIEWS] [COLLABORATORS] [OPENINGS] [TEACHING]
[CONTACT] [SUPORT LUNG
CANCER RESEARCH / DONAR SUPORT RECERCA
CÀNCER DE PULMÓ]
|
![]()
|
LAB MEMBERS |
|||||||||||||||||||||||||
|
Jordi Alcaraz
(Principal Investigator) |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Spain · Education: - Ph.D. in Biophysics,
University of Barcelona, Barcelona (Spain) 2002 - M. Sc. in Cell Physiology,
University of Barcelona, Barcelona (Spain) 2000 - B. Sc. in Physics,
University of Barcelona, Barcelona (Spain) 1997 · Biosketch: Jordi is a Serra-Húnter Associate
Professor in the School of Medicine at the University of Barcelona (UB) since
2016. He graduated in Physics in 1997 at the UB, and attended graduate school
at the same university, where he obtained his Ph.D. in the fields of Cellular
Biophysics and Nanobioengineering in 2002. From 2002 until 2007 he was a
joint postdoc between the Cancer Biology Laboratory of Dr Mina J Bissell at the Lawrence Berkeley National Laboratory and the
Single Molecule Biophysics Laboratory of Prof Carlos
Bustamante at
UC Berkeley. During his postdoc he pursued research aiming to understand how
biophysical cues from the tissue microenvironment control differentiation and
cancer progression at the single cell level. He is also an associate group
leader at the Institute for Bioengineering of Catalonia (IBEC) and a core
member of the Functional Unit of Thoracic Tumors at the Hospital Clinic de
Barcelona. · More at: ORCID:0000-0001-7898-1599
|
||||||||||||||||||||||||
|
Office Ph: (+34) 934031148 Lab Ph: (+34) 934039764 Fax: (+34) 934035278 Address: Unitat de Biofísica i Bioenginyeria Facultat de Medicina
Universitat de Barcelona Casanova 143 08036 Barcelona, Spain |
|||||||||||||||||||||||||
|
Graduate students
(director/tutor) |
|||||||||||||||||||||||||
|
Paula Duch |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Spain · Education: - M. Sc. in Biomedical
Research, University Pompeu Fabra, Barcelona (Spain) 2021 - B. Sc. in Biotechnology, Universidad
de Oviedo, Oviedo (Spain), 2020
|
||||||||||||||||||||||||
|
(+34) 934039764 |
|
||||||||||||||||||||||||
|
Victoria Batto |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Argentina/Spain · Education: - M. Sc. in Biotecnology, Universidad
de Buenos Aires, Buenos Aires (Argentina), 2022 - B. Sc. In Chemical
Sciences, Universidad de Buenos Aires, Buenos Aires (Argentina), 2017
|
||||||||||||||||||||||||
|
(+34) 934039764 |
|
||||||||||||||||||||||||
|
Teia Vallès |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Spain · Education: - M. Sc. in TraBiomedicine,
University of Barcelona, Barcelona (Spain) 2018 - B. Sc. in Biomedical
Sciences, , University of Barcelona, Barcelona (Spain) 2023
|
||||||||||||||||||||||||
|
(+34) 934039764 |
|
||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||
|
Elba Marín |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Spain · Education: - M. Sc. in Translational
Medicine, University of Barcelona, Barcelona (Spain) 2018 - B. Sc. in Biomedical
Sciences, , University of Barcelona, Barcelona (Spain) 2017
|
||||||||||||||||||||||||
|
(+34) 934039764 |
|
||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||
|
Postdoctoral Researchers |
|||||||||||||||||||||||||
|
Patricia Fernández |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Spain · Education: - PhD in Biomedicine, University
of Barcelona, Barcelona (Spain) 2016
- M. Sc. in Biomedicine,
University of Barcelona, Barcelona (Spain) 2010 - B. Sc. in Biochemistry, Autonomous
University of Barcelona, Barcelona (Spain) 2009 - B. Sc. in Biology, Autonomous
University of Barcelona, Barcelona (Spain) 2008 |
||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
(+34)
934039764 |
|
||||||||||||||||||||||||
|
Natalia Díaz |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Chile/Spain · Education: - PhD in Biochemistry, Universidad
de Chile, Santiago (Chile) 2016 - M. Sc. in Biochemistry, Pontificia
Universidad Católica de Valparaíso, Valparaíso (Chile) 2010 - B. Sc. In Science, Pontificia
Universidad Católica de Valparaíso, Valparaíso (Chile) 2007 |
||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
(+34)
934039764 |
|
||||||||||||||||||||||||
|
Marc Rico-Pastó |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Spain · Education: - PhD in Physics, University
of Barcelona, Barcelona (Spain) 2022 - M. Sc. in Photonics,
Polytechnic University of Catalonia, Barcelona (Spain) 2014 - B. Sc. in Physics, University
of Barcelona, Barcelona (Spain) 2013 |
||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
(+34)
934039764 |
|
||||||||||||||||||||||||
|
Marselina Arshakyan |
|||||||||||||||||||||||||
|
|
Brief CV · Citizenship: Armenia · Education: - PhD in Biochemical and
Pharmacological Methodologies, University of Urbino Carlo Bo, Urbino (Italy)
2015 - Master's degree in
Pharmaceutical Chemistry, Pharmaceutics, Yerevan State University, Yerevan
(Armenia) 2004 |
||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
(+34)
934039764 |
|
||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
Past Members |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
![]()
|
|
RESEARCH
Current research projects and long-term research interests
Aberrant cancer-stroma interactions
in lung cancer Lung cancer remains the leading cause of cancer-related
deaths worldwide, with a 5-year survival rate of only 23% that is much lower
than other leading cancer types like breast (91%) or colon (63%). Lung tumors
and other solid neoplasias are increasingly regarded as organs driven by the
aberrant co-evolution of cancer and stromal cells. Based on the striking
similarities between the stroma in tumors and wounds, tumors are often
described as “wounds that never heal”,
and the desmoplastic (wound-like) stroma is pointed as a major contributor to
virtually all steps of tumor progression, including metastasis formation, and
even resistance to therapies. However, the mechanisms underlying the effects
of such tumor stroma on tumor/metastasis-promotion and modulation
of therapy responses, including immune checkpoint inhibitors, remain
poorly understood, particularly in lung cancer. To address this limitation,
we started in 2010 a collection of tumor associated fibroblasts (TAFs)
-the most abundant stromal cell type- from surgical patients of the Hospital
Clínic de Barcelona diagnosed with non-small cell lung cancer (NSCLC), which
is the most abundant lung cancer type. Likewise, we gathered biopsies from a
cohort of primary tumors and paired brain metastasis to unravel the presence
of TAFs in the common dissemination of lung tumors to the brain. We use advanced pre-clinical culture models
to study the aberrant interactions between TAFs and cancer cells or other
stromal cells (immune cells, endothelial cells) in lung cancer to unveil key
molecular alterations that can be useful to develop novel therapies, to identify novel
biomarkers and to dissect resistance mechanisms to current
therapies. Role of abnormal tissue mechanics in
fibrosis and cancer It is well known that each tissue and organ in our body is
characterized by a specific deformability or “stiffness”. Thus, our brain or
lungs are soft organs, whereas our muscle and bones are stiff. In normal
conditions, the stiffness of each tissue is maintained within its
physiological range during adulthood. Occasionally, a region of a tissue may
temporarily stiffen as part of the normal wound healing response to damage. Likewise, tissue stiffness
becomes progressively altered during aging.
None of these previously mentioned mechanical alterations compromise neither
the integrity nor the normal function of the tissue. In contrast, a hallmark
of numerous diseases is the permanent loss of normal tissue stiffness,
concomitantly with an impairment of normal functions. In some cases, the
tissue becomes globally more stiff
as in sclerosis, fibrosis and cancer. In cancer, tissue stiffening has been associated with the
excessive abundance of activated TAFs and subsequent deposition of fibrillar
collagens. Intriguingly, a fraction of cancer cells may become abnormally
soft and hyperflexible, eliciting a mechanical advantage to promote
dissemination. We are particularly interested in how normal tissue stiffness
is lost in fibrosis and cancer, how this abnormal tissue hardening
contributes to the progression of these devastating diseases or resistance to
therapies, and how some cancer cells acquire an hyperflexible phenotype to
enhance their dissemination. Moreover, we are interested in using tissue
mechanics-associated features as novel diagnostic and/or prognostic
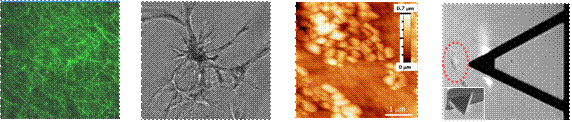
biomarkers. To pursue these interests, we use advanced models based on biomaterials with tunable elasticity and microfluidics as well as Atomic force microscopy (AFM)
and other nanomechanical tools that enable measuring cell and tissue
mechanics with high resolution. Understanding how the collagenous ECM
contributes to tumor progression and formation of brain metastasis We previously reported that the high deposition of
fibrillar collagens is associated with poor prognosis independently of the
TNM staging in NSCLC. However, how the collagen-rich ECM contributes to tumor
progression remains poorly understood. We are interested in understanding how
the collagenous stroma promotes immunosuppression and the acquisition of an
invasive phenotype, both in the context of the primary tumor and brain
metastasis, which is a common metastatic organ in NSCLC. Experimental approaches and areas of expertise
Our research is intrinsically multidisciplinary, as it integrates tools and techniques from a
variety of scientific fields including molecular
and cell biology, biomaterials,
nanobiotechnology and biophysics. The sources of our cells (both human and rodent) are
either primary culture from donors
or commercially available cell lines.
We have also developed an ex-vivo assay based on precision cut thin slices of
fresh tumors from surgical lung cancer patients to directly test patient
response to selected drugs. The main techniques we use in our research include the
following (but are not restricted to): · Cell
culture: primary culture of fibroblasts from tissue explants, culture of
cell lines of mesenchymal or epithelial origin · Genetic
tools for transcription manipulation: shRNA, siRNA · Biomaterials:
2D and 3D gel assays in which both the biochemical composition and the
mechanical properties can be controlled independently · Molecular
cell biology: qRT-PCR, Western-Blotting, Immunofluorescence,
Immunohistochemistry, Zymmography, Flow Cytometry, ELISA · Tools
to model the hydrodynamic features within the circulation:
micropatterning · Advanced
optical microscopy: phase contrast microscopy, DIC, epifluorescence, polarized
light microscopy, confocal microscopy and confocal reflection microscopy · Image
processing with Image J, quPATH, Matlab · Digital
Pathology, with customized software to process histologic stainings from
patients (in bright-field and immunofluorescence) and collagen architecture
(with CT-FIRE) · Bioinformatic
analysis, including analysis of gene expression datasets available at
TCGA, scRNAseq or other databases, pathway enrichment analysis, interactome
analysis etc. · Nano-
Microrheology (i.e. characterization of mechanical properties of soft
samples, including cells, gels and tissues) with Atomic Force Microscopy ·
Theoretical Physics: Soft Condensed Matter and Contact Mechanics |
UP
![]()
CURRENT AND
PAST FUNDING SOURCES (competitive calls)
• Agencia Estatal de Investigación AEI,
Spain
• Instituto de Salud Carlos III ISCIII,
Spain
• Asociación Española Contra el Cáncer
AECC, Spain
• FET-OPEN, Horizon 2020, European
Union
• ERA-NET Transcan-3, European Union
• Sociedad Española de Neumología y
Cirugía Torácica SEPAR, Spain
![]()
PUBLICATIONS
Alejandro Rosell, Agata A Krygowska, Marta Alcón Pérez, Cristina Cuesta,
Mathieu-Benoit Voisin, Juan de Paz, Héctor Sanz-Fraile, Vinothini Rajeeve,
Alberto Berral-González, Ana Carreras-González, Ottilie Swinyard, Enrique
Gabandé-Rodriguez, Julian Downward, Jordi Alcaraz, Juan Anguita,
Carmen García-Macías, Javier De Las Rivas, Pedro Cutillas, Esther Castellano.
AS-p110a signalling
in macrophages is required for effective inflammatory response and resolution
of inflammation. eLife 2024 13:RP94590.
doi.org/10.7554/eLife.94590.2 LINK P. Duch,
N. Díaz-Valdivia, M. Gabasa, R. Ikemori, M. Arshakyan, P. Fernández-Nogueira,
A. Llorente, C. Teixido, J. Ramírez, J. Pereda, L. Chuliá-Peris, J. M.
Galbis, F. Hilberg, N. Reguart, D.C. Radisky, J. Alcaraz. Aberrant
TIMP-1 production in tumor-associated fibroblasts drives the selective
therapeutic effects of nintedanib in lung adenocarcinoma. Cancer Science 2024
115(5):1505-1519 PMID: 38476010 DOI: 10.1111/cas.16141 LINK M. Narciso, Á. Martínez, C. Júnior, N. Díaz-Valdivia, A. Ulldemolins, M.
Berardi, K. Neal, D. Navajas, R. Farré, J. Alcaraz, I. Almendros, N.
Gavara. Lung Micrometastases Display ECM Depletion
and Softening While Macrometastases Are 30-Fold Stiffer and Enriched in
Fibronectin. Cancers (Basel) 2023 15(8):2404. doi:
10.3390/cancers15082404. PMID: 37190331 LINK E. Almici, M.
Arshakyan, J.Ll Carrasco, A. Martínez, J. Ramírez, A. B. Enguita, E. Monsó,
J. Montero, J. Samitier, J. Alcaraz. Quantitative image analysis of
fibrillar collagens reveals novel diagnostic and prognostic biomarkers and
histotype-dependent aberrant mechanobiology in lung cancer. 2023 Mod
Pathol. 2023 Jul;36(7):100155. doi: 10.1016/j.modpat.2023.100155. PMID:
36918057 LINK Y.
Juste-Lanas, N. Díaz-Valdivia, A. Llorente, R. Ikemori, A. Bernardo, M.
Arshakyan, C. Borau, J. Ramírez, J.C. Ruffinelli, E. Nadal, N. Reguart, J. M.
García-Aznar, J. Alcaraz (corresponding). 3D collagen migration
patterns reveal a SMAD3-dependent and TGF-β1-independent mechanism of
recruitment for tumor associated fibroblasts in lung adenocarcinoma. Br J
Cancer 2023 128(6):967-981 2022
(doi 10.1038/s41416-022-02093-x) PMID: 36572730 LINK P.
Duch., N. Díaz-Valdivia, R. Ikemori, M. Gabasa, E.S. Radisky, M. Arshakyan,
S. Gea-Sorlí, A. Mateu, P..Bragado, H. Mori, J. Ramírez, C. Teixidó, N.
Reguart, C..Fillat, D. Radisky, J. Alcaraz. Aberrant TIMP-1
overexpression in tumor-associated fibroblasts drives tumor progression
through CD63 in lung adenocarcinoma. Matrix Biology 2022, 111:
207-225 PMID: 35787446 LINK Lourdes Chuliá-Peris,
Cristina Carreres-Rey, Marta Gabasa, Jordi Alcaraz, Julián Carretero,
Javier Pereda. Matrix
metalloproteinases and their inhibitors in pulmonary fibrosis: EMMPRIN/CD147
comes into play. Int. J. Mol. Sci. 2022, 23(13):6894 DOI:
10.3390/ijms23136894 LINK J. Alcaraz, R.
Ikemori, A. Llorente, N. Díaz-Valdivia, N. Reguart, M. Vizoso. Epigenetic
reprogramming of tumor-associated fibroblasts in lung cancer: therapeutic
opportunities. Cancers (Basel) 2021, 13:3782-3802. DOI: 10.3390/cancers13153782 LINK M. Gabasa, E.
Radisky, R. Ikemori, G. Bertolini, M. Arshakyan., A. Hockla, P. Duch., O.
Rondinone, A. Llorente, M. Maqueda, A. Davalos, E. Gavilán, A. Perera, J.
Ramírez, P. Gascón, N. Reguart, L. Roz., D.C. Radisky, J. Alcaraz.
MMP1 drives tumor progression in large cell carcinoma of the lung through
fibroblast senescence. Cancer Letters 2021, 507:1-12 PMID: 33684534 LINK M.
Gabasa; M. Arshakyan; A. Llorente; L. Chuliá-Peris; I. Pavelescu; A. Xaubet;
J. Pereda; J. Alcaraz. Interleukin-1β Modulation of the
Mechanobiology of Primary Human Pulmonary Fibroblasts: Potential Implications
in Lung Repair. Int. J. Mol. Sci. 2020, 21, 8417 LINK Epigenetic
SMAD3 repression in tumor-associated fibroblasts impairs fibrosis and
response to the antifibrotic drug nintedanib in lung squamous cell carcinoma.
R. Ikemori, M. Gabasa, P. Duch, M. Vizoso, P. Bragado, M. Arshakyan, I-C.
Benchea, A. Marín, S. Morán, M. Castro, G. Fuster, S. Gea-Sorli, T. Jauset,
L. Soucek, L.M. Montuenga, M. Esteller, E. Monsó, V.I. Peinado, P. Gascón, C.
Fillat, F. Hilberg, N. Reguart, J. Alcaraz. Cancer Res 2020 80:276-290 LINK J. Alcaraz, J. Lluís Carrasco, L. Millares, I-C. Luis, F.J.
Fernández-Porras, A. Martinez-Romero, N. Diaz-Valdivia, J. Sanchez De Cos, R.
Rami-Porta, L. Seijo, J. Ramírez, M.J. Pajares, N. Reguart, E. Barreiro, E.
Monsó. Stromal markers of
activated tumor associated fibroblasts predict poor survival and are
associated with necrosis in non-small cell lung cancer. Lung Cancer 2019, 135: 151–160 LINK Esther Marhuenda,
Noelia Campillo, Marta Gabasa, Miguel Angel Martínez-García, Francisco
Campos-Rodríguez, David Gozal, Daniel Navajas, Jordi Alcaraz, Ramon
Farré, Isaac Almendros. Effects
of Sustained and Intermittent Hypoxia on Human Lung Cancer Cells. American Journal of Respiratory Cell and
Molecular Biology 2019;61(4):540-544 LINK Laura Sala, Héctor
Franco-Valls, Jelena Stanisavljevic, Josue Curto, Jordi Vergés, Raúl Peña,
Paula Duch, Jordi
Alcaraz, Antonio G. de Herreros and Josep Baulida. Abrogation of
myofibroblast activities in metastasis and fibrosis by methyltransferase
inhibition. International Journal of
Cancer 2019 145:3064-3077
doi: 10.1002/ijc.32376. LINK Characterization
of the elastic properties of extracellular matrix models by atomic force
microscopy. J. Otero, D. Navajas, J. Alcaraz. Methods in Cell Biology:
Cell-derived Matrices Part A 2019 doi.org/10.1016/bs.mcb.2019.11.016 LINK A. Giménez, P. Duch, M. Puig, M. Gabasa, A.
Xaubet, J. Alcaraz. Dysregulated collagen homeostasis by matrix
stiffening and TGF-β1 in fibroblasts from idiopathic pulmonary fibrosis
patients: role of FAK/Akt. International Journal
of Molecular Science 2017, 18(11), 2431; pii: E2431 LINK M. Gabasa, P. Duch, I.
Jorba, A. Giménez, R. Lugo, I. Pavelescu, F. Rodríguez-Pascual, M.
Molina-Molina, A. Xaubet, J. Pereda, J. Alcaraz. Epithelial contribution to the pro-fibrotic
stiff microenvironment and myofibroblast population in lung fibrosis. Molecular Biology of the Cell 2017,
28(26):3741-3755 LINK M. Gabasa, R. Ikemori, F. Hilberg, N.
Reguart, J. Alcaraz. Nintedanib selectively inhibits the activation
and tumor-promoting effects of fibroblasts from lung adenocarcinoma patients.
British Journal of Cancer 2017,
117:1128-1138 LINK A. Labernadie, T.
Kato, A. Brugués, X. Serra-Picamal, S. Derzsi, V. Gonzalez, A.
Elosegui-Artola, J. Alcaraz, P. Roca-Cusachs, E. Sahai, X. Trepat. A
mechanically active heterophilic E-cadherin/N-cadherin adhesion enables
cancer associated fibroblasts to drive cancer cell invasion. Nature Cell
Biology 2017, 19: 224–237 LINK J. Alcaraz, J. Otero, I. Jorba, D. Navajas. Bidirectional
mechanobiology between cells and their local extracellular matrix probed by
atomic force microscopy. Seminars in
Cell and Developmental Biology 2017, S1084-9521(17)30328-2 LINK A.
Giménez, J.J Uriarte, J. Vieyra, D. Navajas, J. Alcaraz. Elastic properties of hydrogels and
decellularized tissue sections used in mechanobiology studies probed by
atomic force microscopy. Microsc Res
Tech. 2017;80:85-96. LINK R. Lugo, M. Gabasa, F. Andriani, M. Puig, F.
Facchinetti, J. Ramírez, A. Gómez-Caro, U. Pastorino, G. Fuster, I.
Almendros, P. Gascón, A. Davalos, N. Reguart, L. Roz, J. Alcaraz. Heterotypic
paracrine signaling drives fibroblast senescence and tumor progression of
large cell carcinoma of the lung. Oncotarget 2016 7(50):82324-82337 LINK M.
Vizoso, M. Puig, F.J. Carmona, M. Maqueda, A. Velásquez, A. Gómez, A.
Labernadie, R. Lugo, M. Gabasa, L.G. Rigat-Brugarolas, X. Trepat, J. Ramírez,
N. Reguart, S. Moran, A. Perera, M. Esteller, J. Alcaraz. Aberrant DNA
methylation in Non Small Cell Lung Cancer associated fibroblasts. Carcinogenesis 2015,
36:1453-63 LINK E. Monsó, L.M. Montuenga, J. Sánchez de Cos, C.
Villena, and Grupo Colaborativo en Cáncer de Pulmón CIBERES-RTICC-SEPAR-Plataforma
Biobanco Pulmonar (J. Alcaraz et al.), Biological Marker Analysis as Part of
the CIBERES-RTIC Cancer-SEPAR Strategic Project on Lung Cancer. Arch
Bronconeumol, 2015 51(9):462-467 LINK V.
Vicens-Zygmunt, S. Estany, A. Colom, A. Montes-Worboys, C. Machahua, A.J.
Sanabria, R. Llatjos, I. Escobar, F. Manresa, J. Dorca, D. Navajas, J.
Alcaraz, M. Molina-Molina. Fibroblast viability and phenotypic changes
within glycated stiffened three-dimensional collagen matrices. Respiratory Research 2015 Jul 1;16:82 LINK M. Puig, R. Lugo, M. Gabasa, A. Giménez, A. Velásquez,
R. Galgoczy, J. Ramírez, A. Gómez-Caro, Ó. Busnadiego, F. Rodríguez-Pascual, P.
Gascón, N. Reguart, J. Alcaraz. Matrix Stiffening and Beta1 integrin
Drive Subtype-specific Fibroblast Accumulation in Lung Cancer. Mol Cancer Res 2015, 13:161-73. LINK R. Galgoczy, I. Pastor, A. Coloma, A. Giménez, F. Mas, J.
Alcaraz. A spectrophotometer-based diffusivity
assay reveals that diffusion hindrance of small molecules in extracellular
matrix gels used in 3D cultures is dominated by viscous effects. Colloids and Surfaces B: Biointerfaces
2014, 120:200-7 LINK Colom
A, Galgoczy R, Almendros I, Xaubet A, Farré R, Alcaraz J. Oxygen
diffusion and consumption in extracellular matrix gels: Implications for
designing three-dimensional cultures. J
Biomed Mater Res A. 2014,
102:2776-84 LINK I.
Acerbi, T. Luque, A. Giménez, M.
Puig, N. Reguart, R. Farré, D.
Navajas, and J. Alcaraz. Integrin-Specific Mechanoresponses to Compression
and Tension Probed by cylindrical Flat-Ended AFM Tips in Lung Cells. PLoS ONE 2012, 7: e32261 LINK Mori H, Lo AT, Inman JL, Alcaraz J, Ghajar CM, Mott JD, Nelson CM,
Chen CS, Zhang H, Bascom JL, Seiki M, Bissell MJ. Transmembrane/cytoplasmic,
rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary
branching morphogenesis via binding to integrin β1. Development. 2013, 140:343-52
LINK I. Acerbi,
T. Luque, A. Giménez, M.
Puig, N. Reguart, R. Farré, D.
Navajas, and J. Alcaraz. Integrin-Specific Mechanoresponses to Compression
and Tension Probed by cylindrical Flat-Ended AFM Tips in Lung Cells. PLoS ONE 2012, 7: e32261 LINK J. Alcaraz, H. Mori, C.M. Ghajar, D. Brownfield, R. Galgoczy and M.J. Bissell. Collective epithelial cell invasion overcomes
mechanical barriers of collagenous extracellular matrix by a narrow tube-like
geometry and MMP14-dependent local softening. Integr. Biol., 2011,
3:1153–1166 LINK J. Alcaraz, H. Mori, C.M. Ghajar, D. Brownfield, R.
Galgoczy and M.J. Bissell. Collective epithelial cell invasion overcomes
mechanical barriers of collagenous extracellular matrix by a narrow tube-like
geometry and MMP14-dependent local softening. Integr. Biol., 2011,
3:1153–1166 LINK J. Alcaraz, R. Xu, H.
Mori, C.M. Nelson, R. Mroue, V.A. Spencer, D. Brownfield, D.C. Radisky, C.
Bustamante, M.J. Bissell. Laminin and
biomimetic extracellular elasticity enhance functional differentiation in
mammary epithelia EMBO J. 2008,
27:2829-2838 LINK P. Roca-Cusachs, J. Alcaraz,
R. Sunyer, J. Samitier, R. Farré, D. Navajas. Micropatterning
of single endothelial cell shape reveals a tight coupling between nuclear
volume in G1 and proliferation. Biophys J, 2008, 94:4984-4995 LINK J. LeBeyec, R.
Xu, S.Y. Moonlee, C.M. Nelson, A. Rizki, J.
Alcaraz, M.J. Bissell. Cell shape regulates global histone
acetylation in human mammary epithelial cells. Exp Cell Res. 2007,313:3066-3075 LINK F. Rico, J. Alcaraz, J.J.
Fredberg, D. Navajas. Nanomechanics
of lung epithelial cells. Int J of Nanotechnology. 2005, 2:180-194 LINK J.
Alcaraz,
C.M. Nelson, M.J. Bissell. Biomechanical Approaches For Studying Integration
of Tissue Structure and Function In Mammary Epithelia. J Mammary Gland
Biol Neoplasia. 2004,
9:361-374 LINK J. Alcaraz, L. Buscemi, X. Trepat, M. Grabulosa, B. Fabry, R Farré, D. Navajas. Microrheology of cultured lung epithelial cells measured with Atomic
Force Microscopy. Biophys J. 2003, 84:2071-79 LINK J. Alcaraz, L. Buscemi, M.
Puig-de-Morales, J. Colchero, A. Baró, D. Navajas. Correction of
Microrheological Measurements of Soft Samples with Atomic Force Microscopy
for the hydrodynamic Drag on the Cantilever. Langmuir. 2002, 18:
716-721 LINK M.
Puig-de-Morales, M. Grabulosa, J.
Alcaraz, J. Mullol, G.N. Maksym,
J.J. Fredberg, D. Navajas. Microrheology of cultured
airway epithelial cells measured by magnetic twisting cytometry with
frequency domain demodulation. J.Appl.Physiol. 2001,
91: 1152-1159 LINK D. Navajas, J. Alcaraz, R.
Peslin, J. Roca, R. Farré. Evaluation of a method
for assessing respiratory mechanics during non-invasive ventilation. Eur. Respir. J. 2000, 16: 704-709 LINK Book chapters J. Alcaraz, P.
Roca-Cusachs. Shape and Mechanical Cues Underlying Cellular Homeostasis in
Soft Organs. Chapter of Cells, Forces and the Microenvironment (English).
Coordinated by C. M. Cuerrier and A.E. Pelling. Published by Pan Stanford and
World Scientiphic Publishing (US), 2015.
ISBN: 978-981-4613-36-1 LINK J. Alcaraz. Microscopía de Fuerza Atómica. Chapter of Técnicas en
Histología y Biología celular (Spanish). Coordinated by
L. Montuenga. Published by Elsevier (Spain), 2014, 2024. ISBN: 978-84-458-2520-4 LINK J. Alcaraz. Introducción a las aplicaciones biofarmacológicas y biotecnológicas de la
bioingeniería. Chapter of Biotecnología y Biofármacos. Módulo II
(Spanish). Coordinated by J. Piulats. Published by Plan
Nacional de Formación Continuada. Consejo General de Colegios Oficiales de
Farmacéuticos (Spain). 2010. ISBN: 978-84-693-1789-1 Legal Deposit:
M-20075-2010 |
UP
![]()
CURRENT AND PAST COLLABORATIONS WITH PHARMA and
BIOENGINEERING COMPANIES
(* funded projects)
(+ drugs or reagents provided)
• Boheringer-Ingelheim Inc., Austria (*, +)
• Basilea Pharmaceutica, Switzerland (*, +)
• Artidis, Switzerland (*)
• Peptomyc, Spain (+)
• Alentis Therapeutics, Switzerland (*,+)
• Nordic Biosciences, Denmark (+)
• NextCure, US (+)
![]()
NEWS AND VIEWS
ON OUR WORK
New recruitment mechanisms for cancer-associated
fibroblasts in lung adenocarcinoma, 2023 LINK Collagen as a novel diagnostic and prognostic biomarker
independent of TNM in lung cancer, 2023 LINK TIMP-1 as a new therapeutic target in lung adenocarcinoma,
2022 LINK How smoking impact on TGF-b signaling in fibroblasts mediates
resistance to multi-tyrosine kinase inhibitor Nintedanib in Lung Cancer
(collaboration with Boehringer-Ingelheim, Austria), 2019 LINK How fibroblasts modulate response to multi-tyrosine kinase
inhibitor Nintedanib in Lung Cancer (collaboration with Boehringer-Ingelheim,
Spain), 2015 LINK Crowdfunding campaing: “New approaches in the fight
against lung cancer”, 2014 LINK Cover of the 12th issue of the journal of the Royal Society of Chemistry iBiology displays an
image from our iBiology 2011 paper LINK Nomination of our EMBO
J 2008 by the Faculty of 1000 Biology LINK |
![]()
![]()
COLLABORATORS
· Ongoing long-term
collaborations (in alphabetic order):
Oriol Casanovas, ICO-IDIBELL,
Barcelona (Spain) Cristina Fillat, IDIBPAS, Barcelona
(Spain) Luis Montuenga, CIMA, Pamplona (Spain) Josep Ramírez, Hospital Clínic,
Barcelona (Spain) Noemi Reguart, IDIBAPS, Barcelona
(Spain) Luca Roz, Istituto Nazionale dei
Tumori, Milano (Italy) Derek Radisky, Mayo Clinic, Jacksonville
FL (US) Xavier Trepat, IBEC, Barcelona
(Spain) Josep Samitier, IBEC, Barcelona
(Spain) · Clinical & Biomedical Institutions Functional Unit
of Thoracic Tumors, Hospital Clínic, Barcelona (Spain) Institute for
Bioengineering of Catalonia (IBEC), Barcelona (Spain) CIBERES |
![]()
DONAR SUPORT RECERCA CÀNCER DE PULMÓ
SUPPORT LUNG CANCER RESEARCH
El nostre grup de recerca accepta
aportacions voluntàries per ajudar a finançar la seva recerca en càncer de
pulmó.
Per fer-ho, es prega contactar amb
l’investigador principal del grup (Jordi Alcaraz) per email (jalcaraz@ub.edu) o telèfon (93 403 1148). Així mateix, podeu també contactar-nos
per a qualsevol dubte o inquietud relacionada amb la nostra recerca!
Podeu contribuir amb qualsevol
aportació o fent difusió de la nostra recerca.
Moltes gràcies!
-------------------------------------------------------------------------------------------------------------------------------
Our research group accepts volunteer
donations to support our lung cancer research. For this purpose, please contact
the principal investigator of this group (Jordi Alcaraz) by email (jalcaraz@ub.edu) or telephone (+3493 403 1148). Likewise, should you have any doubt or different
request on our research, please contact us!
You can help us by making a donation or
spreading the word of our research.
Many thanks!
![]()
TEACHING
We are actively involved in the
following academic programs: · Degree in
Medicine, UB · Degree in
Molecular Medicine, UB · Degree in
Biomedical Engineering, UB · Master in Biomedicine, UB |
![]()
OPENINGS
FOR MASTER STUDENTS, GRAD STUDENTS AND POSTDOCS
Our group is continuously open to students at the level of
Master, Graduate (PhD) and Postdoctoral level. Please contact us to
inquire about currently available positions and projects. |
![]()
|
GROUP PICTURES |
|
|
ECM Pharmacology Congress, Copenhage
2024
|
Aseica meeting, Zaragoza 2024
|
|
Group lunch, Spring 2023
|
Celebrating Paula´s paper, July 2022
|
|
Celebrating Rafa´s paper, Autumn 2020
|
Lab picture
|
|
Lab picture 2022
|
|
|
Derek Radisky visit, Spring 2019
|
Lab picture 2017
|
|
Crowdfunding 2013
|
|
|
Christmas 2010
|
Christmas 2010
|
![]()
CONTACT
· email · Phone [Office Ph: (+34) 93 403 1148] [Lab Ph: (+34) 934039764] · Office and Lab location Unitat de Biofísica i Bioenginyeria 5th floor, Facultat de Medicina (School of
Medicine) Casanova 143, Barcelona |
![]()